Food Studies features the voices of 11 volunteer student bloggers from a variety of different food- and agriculture-related programs at universities around the world. You can explore the full series here.
 Photo: Mitchell MattesOne of the many reasons I enjoy food science courses is that they make all the abstract concepts I encountered in organic chemistry and biochemistry, semesters ago, suddenly seem quite down-to-earth, and extremely important.
Photo: Mitchell MattesOne of the many reasons I enjoy food science courses is that they make all the abstract concepts I encountered in organic chemistry and biochemistry, semesters ago, suddenly seem quite down-to-earth, and extremely important.
For example, in most of the chemistry and biology courses, we’ve discussed water early in the semester. In chemistry, water is considered the universal solvent because of its molecular structure and interaction capabilities. In biology, we learn that water is vital for all known forms of life. Through the lens of a food scientist, however, water presents much more gripping topics of discussion — such as the crispy vs. chewy chocolate chip cookie debate.
Water is used in food processing as a medium for temperature exchange (boiling, blanching, steaming, etc.) and as a vehicle for transportation around a factory for foods that float (for example, apples). It’s also essential in any cleaning or soaking procedure, as when processing everything from potatoes to coffee beans. But the chocolate chip cookie conversation involves something that I learned in class just a few days ago: the idea of dealing with the water that is already inside of food.
Some of that water is bound or associated with various other molecules, and no longer behaves like pure water. Water activity is the term used by food scientists to express the amount of unbound water — water that still behaves like water — that is available for chemical and biological reactions.
Why is this important, you ask? Well, primarily because of the role water activity plays in a food’s shelf life. If a product is kept below a certain water activity level, microbial growth is inhibited, which results in a longer shelf life. Also, in mixtures of food types with drastically different water activities — say raisins (moderate water activity) and bran flakes (low water activity) in breakfast cereal — over time, the water from the raisins will travel to the bran flakes. So food scientists can use water activity to determine how much moisture migration will affect their product.
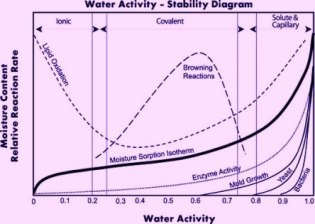 As you might expect, water activity is very much related to overall moisture content. In class, we went into detail about every nuance in their somewhat complicated graphical relationship, but to generalize, a food that is dry, hard, and crisp has a low moisture content and low water activity, and a food that is moist, sticky, and soft, has a high moisture content and high water activity. Where things get really interesting is in thinking about how to transition a food from soft and sticky to dry and crispy, or vice versa.
As you might expect, water activity is very much related to overall moisture content. In class, we went into detail about every nuance in their somewhat complicated graphical relationship, but to generalize, a food that is dry, hard, and crisp has a low moisture content and low water activity, and a food that is moist, sticky, and soft, has a high moisture content and high water activity. Where things get really interesting is in thinking about how to transition a food from soft and sticky to dry and crispy, or vice versa.
Take chocolate chip cookies, for example. The range of possible moisture contents is vast. They can be cakey and chewy, or brittle and crumbly, or anything in between. Yet food scientists can actually account for a cookie’s textural attributes based on a scientific understanding of water activity and moisture content. It’s not just about adding more or less liquid to cookie dough to generate a moister or drier product — that’s only half of the story. It’s really all about adding more of another ingredient that will bind or trap more water!
That other ingredient — for cookies, at least — is most commonly a form of sweetener, such as white sugar, brown sugar, high-fructose corn syrup, or molasses. In other words, adding more sweetener to your cookie traps more moisture, which in turn creates a chewier texture. (At this point, you should feel free to replicate our classroom experiment, and verify for yourself that chewy chocolate chip cookies do indeed taste much sweeter than crunchy ones.)
Meanwhile, the same migration issue that occurs due to the varying moisture activities of raisins and bran flakes also applies to cookies and their interaction with the air. Chewy cookies are actually moister than the air and crunchy cookies are drier than the air. As in all scientific reactions, equilibrium is desired, so moisture from the chewy cookies will want to leave and enter the air, while some of the moisture from the air will want to enter a crunchy cookie. This is why things go stale — why chewy things become hard and crispy things become soft! Hence the need for all of that fancy resalable packaging comes in, to create a waterproof barrier between the cookie and the atmosphere.
Now, imagine yourself working at a cookie factory or a bakery — or even making cookies from scratch at home — and realizing how this one little concept can affect your recipe design and the likelihood of achieving the final product you desire. I know that, personally, the search for a recipe that successfully creates my idea of the perfect chocolate chip cookie is still ongoing, but with this new knowledge in hand, I’ll be tweaking the amount of sugar and other water-binding molecules present in the standard Toll House recipe to create the texture and moisture content I have always dreamed of.