
Should I stay or should I go?
SUPER DUPER UNBELIEVEABLE UPDATE: The AP reports that the “Smart Choices” label is taking a break:
The Smart Choices nutrition labeling program, created voluntarily by nine large U.S. manufacturers, is halting after federal regulators said such systems could mislead consumers, officials with the labeling group said Friday.
… Officials with Smart Choices in Washington, D.C., said Friday that the group will “postpone” active operations and not encourage wider use of the logo while the FDA investigates labeling issues.
However, products that currently use the label are able, but not obligated, to keep using it. I admit to being surprised. May this be the first of many victories over Big Food’s worst excesses. Of course, we may not have seen the last of the “Smart Choices” label, but at least when and if it returns, it will do so under greater FDA regulation.
Reports are flowing in that, in response to Big Food’s attempt to make Froot Loops a “Smart Choice,” the FDA is fast-tracking its own efforts to set groundrules for front-of-packaging nutrition labels. This in itself is a big deal. Avoiding FDA entanglements was the entire point of large food companies coalescing around the “Smart Choices” label. The problem for them is that any real nutrition label might actually depress sales on some products. That is not an option, and so we are given, in Lake Wobegon style (“where all children are above average”), a “healthy” label for which just about every product, no matter how sweet or salty or processed into plasticity, could qualify.
If you’re a Big Food exec, this is bad enough. But, according to the NYT, things just got much worse. Buried in their article on the FDA announcement is this nugget:
Speaking in a telephone call with reporters, Dr. Hamburg said that she expected package-front labels would be required to include information on saturated fat, salt, added sugar and calories.
Dr. Hamburg repeatedly mentioned a package-front labeling program in Britain that uses red, yellow or green dots — like traffic signals — to indicate the relative amounts of important ingredients.
She said that could provide a model for the F.D.A. as it sought to find the best way to provide information to American consumers.
Uh oh. The industry-despised “traffic signals” label. The UK’s version looks like this:
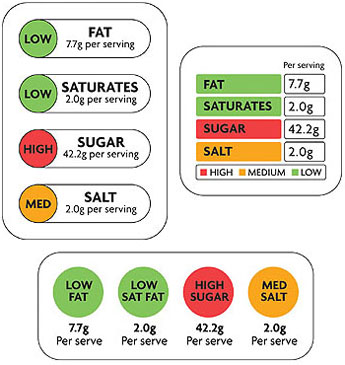
There are two problems with them from the industry’s perspective. The first, of course, is that there will be red lights!! The supermarket will suddenly be filled with product after product covered in nasty little red dots that the government essentially says you shouldn’t buy (except once in a while). Sales of products heavy in saturated fat, salt and added sugar will likely, dare I say it, fall.
The second problem is that, according to a study from Australia, where the system is also in place, traffic signal labels are really really effective:
Australian researchers tested four labelling systems and found that product comparisons could be made quickly and easily under the traffic light (TL) system, according to the study…
The 790 consumers surveyed also indicated a reduced intention to purchase products with red and amber nutrient classifications – green being most healthy.
Obviously, there will be some amount of reformulating and reducing of unhealthy ingredients by industry in a struggle to change some of those red lights to yellow (or even green). But products that are 40% sugar by weight like Froot Loops? There will be a big old red light on that sucker.
Now there’s no guarantee the FDA will end up proposing a traffic light system. But Hamburg’s early endorsement of such a system is enough to give Big Food executives heartburn. We need to see where all this ends up, but the noises coming from Hamburg and her number two, Joshua Sharfstein, on this and other issues are music to my ears. Perhaps we can expect great things from the FDA over the next few years. Wouldn’t that be a nice change?



